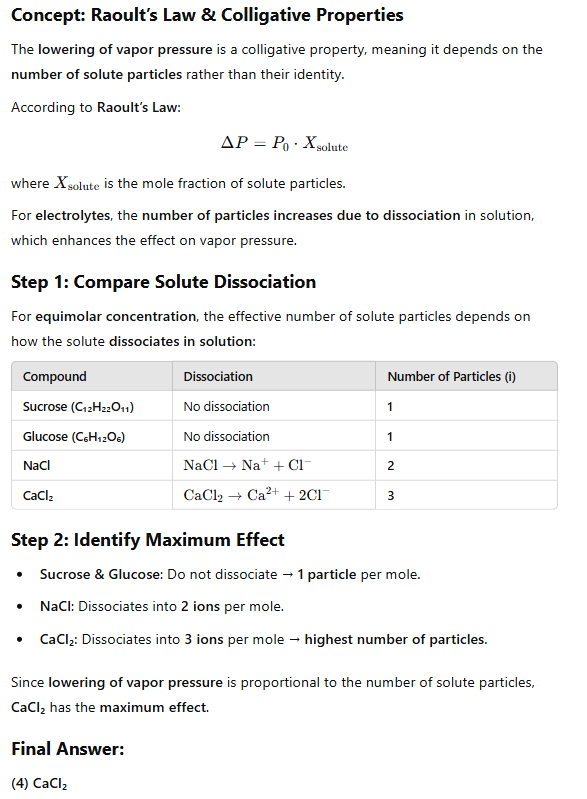
Which of the following will have maximum effect on lowering in vapour pressure if added in equimolar concentration?
(1) Sucrose
(3) NaCl
(2) Glucose
(4) CaCl₂
What is Vapor Pressure Lowering?
When you add a solute (like sugar or salt) to a solvent (like water), it lowers the vapor pressure of the solvent. This is called lowering of vapor pressure, and it’s one of the colligative properties. Colligative properties depend on how many particles are dissolved in the solution, not what those particles are.
Key Concept: Van’t Hoff Factor (i)
The Van’t Hoff factor (i) is the number of particles that a solute breaks into when it dissolves in the solvent. Here’s why it matters:
- If a solute dissociates (breaks into ions), it increases the number of particles in the solution, which means a bigger effect on lowering the vapor pressure.
- If a solute does not dissociate, it will contribute fewer particles to the solution.
Step-by-Step Breakdown:
1. Sucrose (C₁₂H₂₂O₁₁):
- Sucrose is a non-electrolyte, meaning it does not dissociate into ions in water.
- So, i = 1 (it stays as one particle).
2. Glucose (C₆H₁₂O₆):
- Like sucrose, glucose is also a non-electrolyte.
- It also does not dissociate, so i = 1.
3. NaCl (Sodium chloride):
- NaCl dissociates in water into two ions: Na⁺ and Cl⁻.
- So, for NaCl, i = 2 (two particles per formula unit).
4. CaCl₂ (Calcium chloride):
- CaCl₂ dissociates in water into three ions: Ca²⁺ and two Cl⁻ ions.
- So, for CaCl₂, i = 3 (three particles per formula unit).
How Does This Affect Vapor Pressure?
The formula for lowering of vapor pressure is related to the number of solute particles. More particles lead to a greater decrease in vapor pressure.
- Sucrose and Glucose contribute only 1 particle.
- NaCl contributes 2 particles.
- CaCl₂ contributes the most: 3 particles.
Conclusion:
Since CaCl₂ produces the most particles in solution, it will have the strongest effect on lowering the vapor pressure, followed by NaCl, and finally sucrose and glucose, which do not dissociate and produce fewer particles.
So, the answer is (4) CaCl₂ because it has the maximum effect on lowering the vapor pressure.
Does that make sense now?