How many millilitres of 0.05 N HCI are required to neutralize eight grams of NaOH?
(1) 5000
(2) 4000
(3) 4500
(4) 5050
To determine the volume of 0.05 N HCl required to neutralize 8 g of NaOH, follow these steps:
Step 1: Calculate Moles of NaOH
- Molecular weight of NaOH = 40 g/mol
- Moles of NaOH = 8 g / 40 g/mol = 0.2 moles
Step 2: Neutralization Reaction
The balanced reaction is:
NaOH+HCl→NaCl+H2O
- 1 mole of NaOH reacts with 1 mole of HCl
- So, 0.2 moles of NaOH requires 0.2 moles of HCl
Step 3: Calculate Equivalent of HCl
- Normality (N) = Molarity × Equivalent factor
- For HCl, Normality = Molarity because it fully ionizes.
- Milliequivalents of HCl required = milliequivalents of NaOH
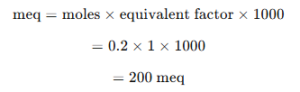
Step 4: Calculate Volume of 0.05 N HCl
Using the formula:
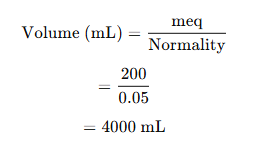
Final Answer:
(2) 4000 mL