If you were to take 1.0 ml of a solution of HCl with a pH of 4.0 and add it to 9.0 ml of distilled water, what would be the pH of the final solution?
(1) The pH would remain unchanged.
(2) The pH would rise to 5.5.
(3) The pH would rise to 5.0.
(4) The pH would rise to 7.0.
To determine the final pH after diluting 1.0 mL of HCl (pH = 4.0) with 9.0 mL of distilled water, follow these steps:
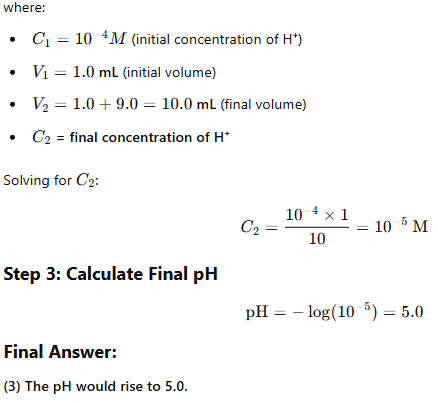
Step 1: Calculate Initial [H⁺] Concentration
pH is given bY
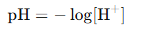
Since pH = 4.0,
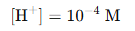
Step 2: Apply Dilution Formula
Using the dilution equation:
C1V1=C2V2