Osmotic pressure does not depend on
(1) Temperature
(3) Molecular weight
(2) Degree of ionization
(4) Molar concentration
Osmotic Pressure:
Osmotic pressure is the pressure required to stop the flow of solvent through a semi-permeable membrane separating two solutions of different concentrations. It depends on the concentration of solute particles in the solution.
Formula for Osmotic Pressure:
The osmotic pressure (π) is given by the formula:
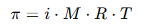
Where:
- i is the Van’t Hoff factor (degree of ionization)
- M is the molar concentration of the solute
- R is the gas constant
- T is the temperature in Kelvin
What does Osmotic Pressure depend on?
- Temperature (T): As per the formula, osmotic pressure increases with temperature because temperature is directly proportional to osmotic pressure.
- Molar concentration (M): Osmotic pressure is directly proportional to the molar concentration of solute particles. More solute = higher osmotic pressure.
- Degree of ionization (i): If a solute ionizes into multiple ions (like NaCl → Na⁺ + Cl⁻), it increases the number of particles in the solution, which increases the osmotic pressure.
What does it not depend on?
- Molecular weight: Osmotic pressure does not depend on the molecular weight of the solute directly. A larger molecule will affect the molar concentration, but osmotic pressure is more influenced by how many particles (ions or molecules) are present, not their size or mass.
Final Answer:
(3) Molecular weight — Osmotic pressure does not depend on the molecular weight of the solute.