The pH of a 0.001 molar HCl solution in H₂O is:
(1) 1
(2) 2
(3) 3
(4) 4
To determine the pH of a 0.001 M HCl solution, follow these steps:
Step 1: Understanding pH Calculation
- HCl is a strong acid, meaning it fully dissociates in water:
![]()
- The concentration of H⁺ ions in solution is equal to the given molarity of HCl.
Step 2: Apply pH Formula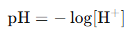
Since [H⁺] = 0.001 M = 10^{-3} M,
![]()
Final Answer:
(3) 3