What will be the required volumes of 1N HCl and 4N NaOH to prepare one litre solution of pH 77
(1) 500 ml, 500 ml.
(3) 600 ml, 400 ml
(2) 800 ml, 200 ml
(4) 200 ml, 800 ml
To prepare 1 litre of a solution with pH = 7 using 1N HCl and 4N NaOH, follow these steps:
Step 1: Understanding pH = 7
- pH = 7 means the solution is neutral.
- This implies that the milliequivalents (meq) of HCl must equal the milliequivalents of NaOH.
Step 2: Define Variables
Let:
- V1V_1V1 = Volume of 1N HCl (in mL)
- V2V_2V2 = Volume of 4N NaOH (in mL)
- Total volume = 1L = 1000 mL
Since the solution is neutral:
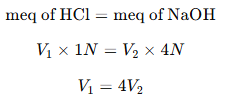
Step 3: Solve for V1V_1V1 and V2V_2V2
Since V1+V2=1000 mL,
Substituting V1=4V2
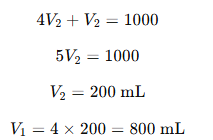
Final Answer:
(2) 800 mL of 1N HCl and 200 mL of 4N NaOH