You are provided with 1.0M solutions of NaCl, (NH_{4}) 2 SO 4 , MgSO4 and K_{3}P*O_{4} In which of these solutions would a protein be expected to be lest soluble?
(1) NaCl
(2) (N*H_{4}) 2 SO 4
(3) MgSO4
(4) K_{3}PO_{4}
The solubility of a protein in a salt solution is influenced by the salting-out effect, which depends on the ionic strength of the solution. The higher the ionic strength, the more the protein tends to precipitate out of solution.
Understanding Ionic Strength:
Ionic strength (I) is given by the formula:

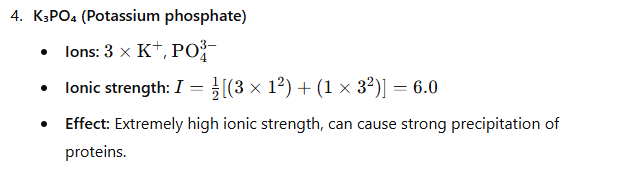
Conclusion:
Since proteins are least soluble in solutions with the highest ionic strength, the correct answer is:
(4) K₃PO₄ (Potassium phosphate)